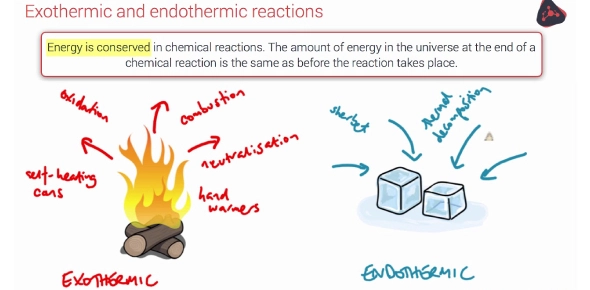
Think you know whether a reaction absorbs or releases heat? Take our Endothermic vs Exothermic Reactions Trivia Quiz to test your chemistry knowledge and find out your expertise level!
Questions and Answers
What's inside the Heat Up Your Knowledge: Endothermic vs Exothermic Reactions Quiz quiz
Which reaction absorbs heat from its surroundings?
What type of reaction releases heat?
Photosynthesis is an example of which type of reaction?
Burning wood is an example of:
Which process requires energy input to proceed?
The reaction of hydrochloric acid with sodium hydroxide is:
Melting ice absorbs heat and is an example of:
Which reaction typically lowers the temperature of its surroundings?
Formation of water from hydrogen and oxygen releases heat. This is a:
Which of the following is not typically associated with exothermic reactions?
Cooling packs used in first aid are based on:
Which reaction type is essential for cellular respiration?
Dissolving ammonium nitrate in water is an example of:
Which of the following indicates an exothermic reaction?
The breakdown of glucose during glycolysis is:
Which process results in the absorption of heat energy?
Quiz description
Test Your Knowledge on Endothermic and Exothermic Reactions
Delve into the fascinating world of chemical reactions with our engaging trivia quiz focused on endothermic and exothermic processes. Whether you're a chemistry student or just passionate about science, this quiz will challenge your understanding of how different reactions absorb and release heat.
What You'll Learn
- The fundamental differences between endothermic and exothermic reactions
- Real-life examples of each reaction type
- How temperature changes indicate the nature of a reaction
Why Take This Quiz?
Understanding these reactions is crucial for various applications, from industrial processes to everyday phenomena like photosynthesis and combustion. Sharpen your chemistry skills and discover how these reactions play a pivotal role in the natural and man-made world.
Quiz Features
- 15 thought-provoking multiple-choice questions
- Detailed explanations for each answer
- A personalized result to gauge your chemistry prowess