Test your knowledge with our 'Mastering the Properties of Matter' quiz! Dive into questions about states, density, and more to see if you're a novice, intermediate, or expert in understanding matter.
Questions and Answers
What's inside the Mastering the Properties of Matter: Are You an Expert? quiz
Which property of matter is defined as the ability to be drawn into a wire?
What term describes the change from a solid directly to a gas without passing through the liquid phase?
What is the term for the amount of mass per unit volume?
What process involves a gas turning into a liquid?
Which property of matter refers to how much matter is packed into a given space?
What is the term for a substance that cannot be broken down into simpler substances?
Which property of matter describes how it responds to applied forces, such as stretching or compressing?
Which property of matter is a measure of the average kinetic energy of its particles?
What process describes the transition from a liquid to a gas?
Which property of matter determines how much light it can reflect?
What term describes a homogeneous mixture of two or more substances?
Which property of matter refers to its ability to conduct electricity?
Quiz description
Understanding the Properties of Matter
The properties of matter are fundamental concepts in science that describe the characteristics and behaviors of different substances. Whether you're a student, educator, or a curious mind, having a solid grasp of these properties is essential for exploring the physical world around us.
States of Matter
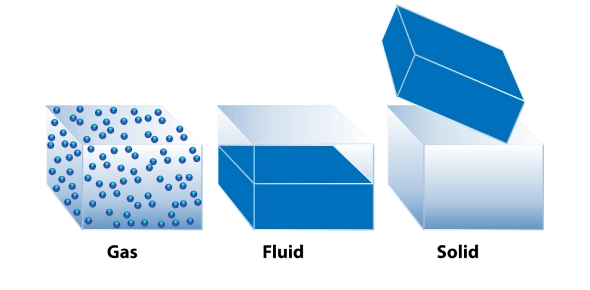
Matter exists in various states, primarily solid, liquid, gas, and plasma. Each state has distinct properties:
- Solid: Definite shape and volume. Particles are tightly packed in a fixed structure.
- Liquid: Definite volume but takes the shape of its container. Particles are less tightly packed and can move past one another.
- Gas: Neither definite shape nor volume. Particles are far apart and move freely.
- Plasma: An ionized state with free electrons and ions. It conducts electricity and is affected by magnetic fields.
Key Properties
Several properties help in identifying and understanding matter:
- Density: Mass per unit volume. High density means more mass in a given space.
- Malleability: Ability to be hammered into thin sheets.
- Ductility: Ability to be drawn into wires.
- Conductivity: Ability to conduct heat and electricity.
- Boiling and Melting Points: Temperatures at which matter changes states.
Chemical vs. Physical Properties
Properties can be classified as chemical or physical:
- Physical Properties: Can be observed without changing the substance's identity, such as color, density, and melting point.
- Chemical Properties: Describe how a substance interacts with other substances, leading to a chemical change, such as reactivity with acids or oxidation.
Applications of Matter Properties
Understanding the properties of matter is crucial in various fields:
- Chemistry: Designing reactions and understanding molecular structures.
- Engineering: Selecting materials based on strength, flexibility, and conductivity.
- Environmental Science: Managing resources and understanding pollution.
- Medicine: Developing pharmaceuticals and medical devices.
By mastering the properties of matter, you can enhance your knowledge and apply it effectively in both academic and real-world scenarios.