Think you know molecular polarity? Take our expert quiz to test your chemistry knowledge and see how well you understand the principles that govern molecular interactions. Challenge yourself today!
Questions and Answers
What's inside the Molecular Polarity Expert Quiz: Test Your Chemistry Mastery quiz
What determines the polarity of a molecule?
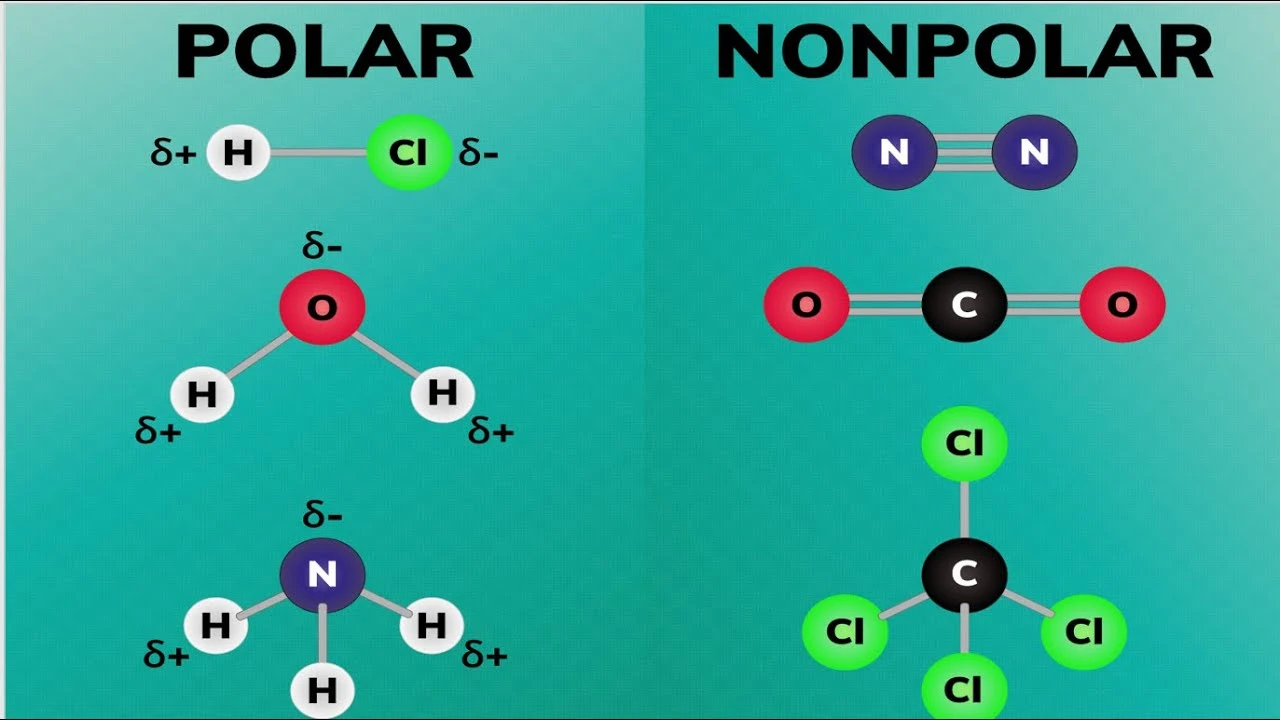
Which molecule is nonpolar?
What shape does a molecule with two bonding pairs and two lone pairs adopt?
Which of the following bonds contributes to molecular polarity?
Why is methane (CH₄) considered nonpolar?
What is the dipole moment of a molecule?
Which molecular geometry typically leads to a nonpolar molecule?
How does molecular polarity affect solubility?
Which of the following is a polar molecule?
What role do lone pairs play in molecular polarity?
Why is ammonia (NH₃) a polar molecule?
Which statement is true about molecular polarity?
What is the polarity of carbon dioxide (CO₂)?
Which of the following affects the polarity of a molecule?
How does symmetry affect molecular polarity?
Which molecule has a net dipole moment?
Quiz description
Enhance Your Understanding of Molecular Polarity
Molecular polarity is a fundamental concept in chemistry that describes the distribution of electrical charge within a molecule. Understanding polarity is crucial for predicting the behavior of substances in various chemical reactions and interactions.
Why Molecular Polarity Matters
Polarity affects many properties of molecules, including solubility, boiling and melting points, and interactions with other molecules. For instance, polar molecules tend to dissolve well in polar solvents like water, while nonpolar molecules are more soluble in nonpolar solvents such as hexane.
Key Factors Influencing Polarity
- Electronegativity: The difference in electronegativity between atoms determines the bond polarity.
- Molecular Geometry: The shape of the molecule affects how dipole moments from individual bonds add up.
- Lone Pairs: Presence of lone electron pairs can create asymmetry, contributing to overall molecular polarity.
Assess Your Knowledge
To deepen your grasp of molecular polarity, take our interactive quiz. It covers various aspects, from identifying polar and nonpolar molecules to understanding the impact of molecular geometry and electronegativity. Challenge yourself and see where you stand!
Benefits of Taking the Quiz
- Identify Strengths and Weaknesses: Understand which areas of molecular polarity you excel in and where you might need more study.
- Enhance Learning: Reinforce your knowledge through targeted questions that cover essential concepts.
- Prepare for Exams: Use the quiz as a study tool to prepare for chemistry exams and assessments.