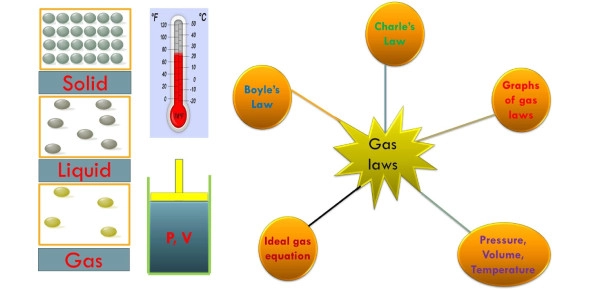
Think you know your gas laws? Take our Ultimate Gas Laws Trivia to test your chemistry knowledge and see how you stack up against others. Challenge yourself with 15 engaging questions covering Boyle's, Charles', and Avogadro's Laws!
Questions and Answers
What's inside the Ultimate Gas Laws Trivia: Test Your Chemistry Knowledge! quiz
Which gas law relates pressure and volume at constant temperature?
According to Charles' Law, what happens to the volume of a gas when temperature increases at constant pressure?
Avogadro's Law states that equal volumes of gases contain an equal number of what, at the same temperature and pressure?
What is the ideal gas law equation?
Which gas law combines Boyle's, Charles', and Avogadro's laws?
At constant volume, how does pressure change with temperature according to Gay-Lussac's Law?
What is Avogadro's number?
If the temperature of a gas is doubled at constant pressure, what happens to its volume according to Charles' Law?
Which variable is directly proportional to the number of moles in the ideal gas law?
What does the 'R' represent in the ideal gas law equation PV = nRT?
According to Boyle's Law, if the volume of a gas is decreased by 30% at constant temperature, what happens to its pressure?
What assumption is made about gas molecules in the ideal gas law?
Which gas law would you use to determine the relationship between pressure and temperature at constant volume?
At constant temperature and pressure, if the number of moles of a gas increases, what happens to the volume?
What is the main difference between real gases and ideal gases?
Quiz description
Welcome to the Ultimate Gas Laws Trivia!
Are you ready to challenge your understanding of the fundamental gas laws that govern the behavior of gases in chemistry? This quiz covers essential principles like Charles' Law, Boyle's Law, and Avogadro's Law, which are crucial for mastering topics in chemistry and related fields.
Why Gas Laws Matter
Gas laws are pivotal in explaining how gases respond to changes in pressure, temperature, and volume. Whether you're a student preparing for exams, a chemistry enthusiast, or someone looking to refresh your scientific knowledge, understanding these laws is indispensable.
What to Expect
- Questions covering key concepts of Boyle's, Charles', and Avogadro's Laws.
- Real-world applications of gas laws in various scientific and industrial contexts.
- Challenging scenarios that test the application of the Ideal Gas Law.
Prepare to Test Your Knowledge
Take this trivia to evaluate how well you grasp the behavior of gases. Whether you're reinforcing classroom learning or simply curious about gas laws, this quiz offers a comprehensive assessment of your chemistry prowess.
Share and Compete
Think you scored high? Share the quiz with friends and see who among you has the superior understanding of gas laws!